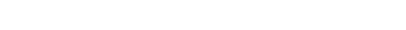π-stacking interactions, conducting organic materials
π-stacking interactions are ubiquitous intermolecular interactions between conjugated molecules. We focus on those particularly close π-stacking interactions that form between two or more radicals, as illustrated above for the dimer of phenalenyl. These stabilizing interactions produce relatively strong and orientation sensitive bonding sometimes called “pancake bonding”. These interactions are particularly well suited for transferring electrons from molecule to molecule and play therefore a key role in organic conductors based on conjugated molecules. Given the exponential increase of the overlap between molecules as the distance is reduced, we are particularly interested in designing new systems with the shortest possible π-stacking contacts.
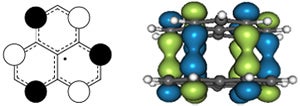
Another consequence of pancake bonding is that π-stacking intermediates can be stabilized by them as illustrated above.
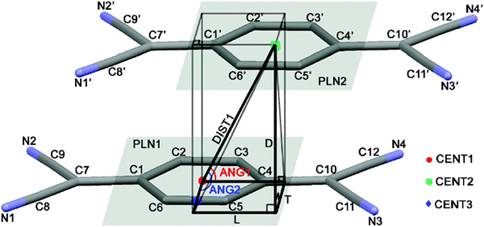
Statistical analysis of thousands of crystal structures containing π-stacking interactions was done using geometrical criteria based on diagrams as shown above. This analysis provided new insights into the packing energetics of TCNQ radical anions. A significant term is obtained by the bonding interaction of the singly occupied molecular orbitals of the TCNQ radical anion as illustrated below:
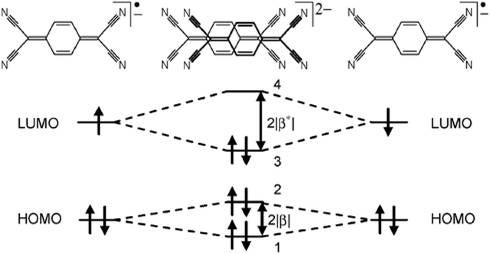
The energy gain associated with the stabilization of the SOMO (the LUMO in this case) is governed by the favorable overlap between to adjacent molecules with significant π-π overlap. At the same time, larger overlap provides for more electron delocalization between repeat units which is essential for electrical conductivity. Similar principles can be applied to the recently discovered neutral radicals based organic conductors from Robert Haddon’s group. An orbital diagram for those compounds are shown below which is the basis of interpreting our calculations on a number of properties of these promising organic conductors.
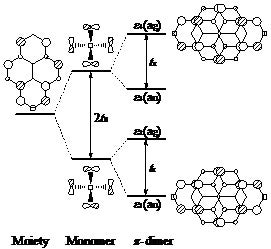
The diagram below deals with a molecule where the p-p overlap occurs between two π-systems within a single molecule providing a testing ground for methods and ideas concerning SOMO-based p-stacking interactions.
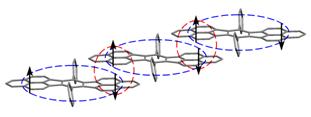
A stepped one-dimensional Kubo π-chain of molecules excised from the crystal structure with interplanar separation of D = 3.14 Å. The antiferromagnetic coupling is illustrated by ellipses (intra- and intermolecular couplings are indicated by horizontally and vertically oriented ellipses, respectively). Orbital interpretation of these systems provides an overall accurate and predictive picture including geometry, energy gap, optical and magnetic properties. These insights are pivotal in designing new organic based conducting and magnetic materials.
Selected references:
- One-Dimensional Metallic Conducting Pathway of Cyclohexyl-Substituted Spiro-Biphenalenyl Neutral Radical Molecular Crystal”
Huang, J.S.; Kertesz, M. J. Am. Chem. Soc. 2006, 128, 1418-1419. - “Stepwise Cope Rearrangement of Cyclo-Biphenalenyl via an Unusual Multicenter Covalent π-Bonded Intermediate”
Huang, J.S.; Kertesz, M. J. Am. Chem. Soc. 2006, 128, 7277-7286. - “Intermolecular Covalent π-π Bonding Interaction Indicated by Bond Distances, Energy Bands, and Magnetism in Biphenalenyl Biradicaloid Molecular Crystal”
Huang, J.; Kertesz, M. J. Am. Chem. Soc. 2007, 129, 1634-1643. - “Crystal Packing of TCNQ Anion π-radicals Governed by Intermolecular Covalent π−π Bonding: DFT Calculations and Statistical Analysis of Crystal Structures”
Jingsong Huang, Stephanie Kingsbury, and Miklos Kertesz, Phys. Chem. Chem. Phys., 2008, 10, 2625–2635. - “Fluxional σ-Bonds of 2,5,8-Tri-tert-butyl-1,3-diazaphenalenyl Dimers: Stepwise [3,3], [5,5] and [7,7] Sigmatropic Rearrangements via p-Dimer Intermediates”, Tian, Y.-H.; Huang, J.S.; Kertesz, M., Phys. Chem. Chem. Phys. 2010, 12, 5084-5093.
- “Bimolecular Hydrogen Transfer in Phenalene by a Stepwise Ene-like Reaction Mechanism” Tian, Y.-H.; Kertesz, M. Chem. Comm. 2010, 46, 4282-4284.
- “Is There a Lower Limit to the CC Bonding Distances in Neutral Radical π-Dimers? The Case of Phenalenyl Derivatives”, Tian, Y.-H.; Kertesz, M. J. Am. Chem. Soc., 2010. 132, 10648-10649.
- “Charge Shift Bonding Concept in Radical pi-Dimers”, Tian, Y.-H.; Kertesz, M. J. Phys. Chem A, 2011, 115, 13942-13949.