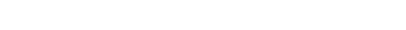Structure and electronic structure of fullerenes and carbon nanotubes; molecules inside nanotubes
Single-walled carbon nanotubes (SWNTs) can serve as nanocontainers encapsulating various molecules, including stable molecules as fullerenes and otherwise unstable molecules, such as carbon chains.
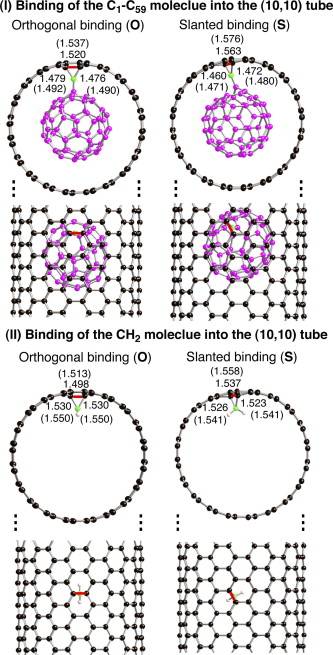
Analogies between the relevant orbitals of simpler and more complex (C 60) reactants have helped to interpret the results of these calculations. We also discovered structural distortion patterns that predict novel substitutional preferences in these nanomaterials.
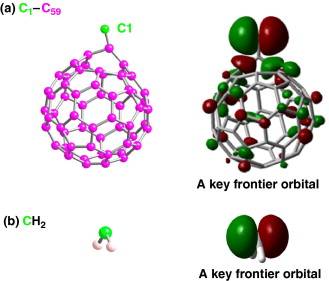
Linear carbon chain is the linear sp hybridized allotropic form of carbon. Its study has become possible inside carbon nanotubes primarily by Raman spectroscopy. We have established its geometry, which is an alternating chain of single and triple bonds based on energetics calculations and the interpretation of the Raman spectra of these chains inside carbon nanotubes. We also obtained evidence from calculated reaction barriers about the preferences for long vs. short encapsulated carbon chains.
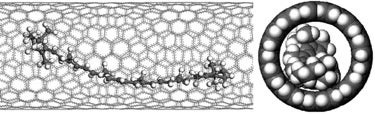
Conformational changes cause detectable shifts in the vibrational modes of many molecules. Conformations of β-carotene molecules encapsulated in single walled carbon nanotubes (car@SWNT) are modeled and evaluated based on experimental Raman spectra from the literature, most notably the emergence of a new band. A combination of experimental Raman spectroscopy data with force field and QM modeling provided conformational information on carotene molecules inside carbon nanotubes.
Selected references:
- “Bond length alternation and charge transfer in a linear carbon chain inside of a single walled carbon nanotube”
Rusznyák, Á. ; Zólyomi, V.; Kürti, J.; Yang, S.; Kertesz, M. Phys. Rev. B. 2005, 72, Art. nu. 155420. - “Cooperative behaviors in carbene additions through local modifications of nanotube surfaces”
Takashi Yumura, Miklos Kertesz, Chem. Mater. 2007, 19, 1028-1034. - “Local Modifications of Single Wall Carbon Nanotubes Induced by Bond Formation with Encapsulated Fullerenes”
Takashi Yumura, Miklos Kertesz, Sumio Iijima, J. Phys. Chem. B. 2007, 111, 1099-1109. - “Linear Cn Clusters: Are They Acetylenic or Cumulenic?“
Yang, S.; Kertesz, M. J. Phys. Chem. A. 2008; 112(1); 146-151. - “Roles of Conformational Restrictions of a Bismalonate in the Interactions with a Carbon Nanotube”
Yumura, T.; Kertesz, M. J. Phys. Chem. C. 2009, 113, 14184-14194. - Energetics of linear carbon chains in one-dimensional restricted environment
Kertesz, M.; Yang, S.J. Phys. Chem. Chem. Phys. 2009, 11, 425-430. - “Conformational Preferences of β–Carotene in the Confined Spaces Inside Carbon Nanotubes” Horn, P.; Kertesz, M. J. Phys. Chem. C, 2010 114, 12139–12144.